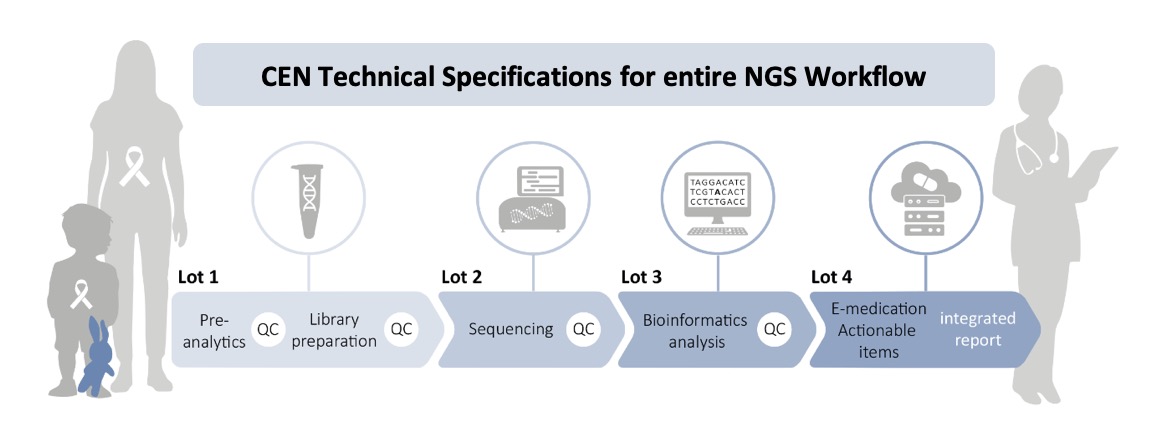
Event Announcement: NGS for Precision Medicine and Pharmacogenomics in Cancer

The Instand NGS4P project consortium and Contractors will meet in Graz on the 9th of April 2024 to present the project and the developed Solutions at a public meeting. Two keynote speakers, Jean-Yves Blay (from Instand-NGS4P Partner CLB) and Matthias Schwab (IKP) will give talks on the challenges of precision medicine of cancer and on