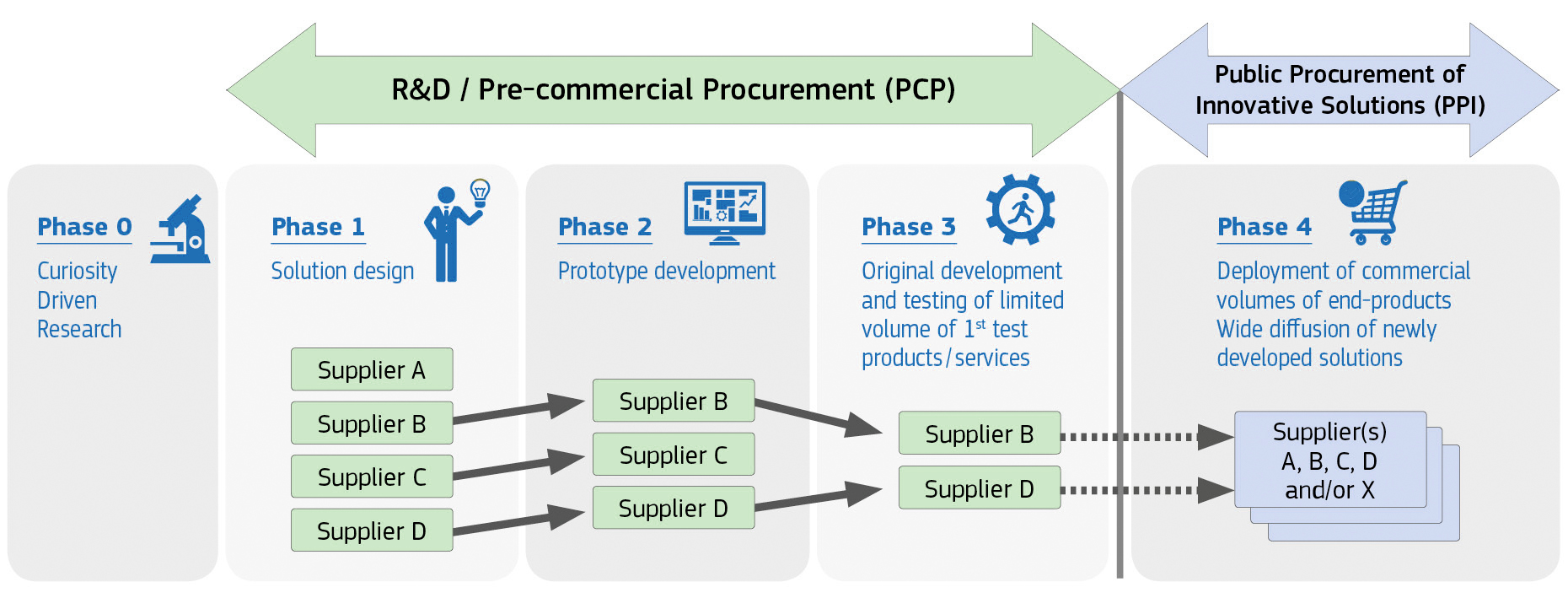
General Information on PCP
The PCP project consists of several sequential phases:
Source: https://ec.europa.eu/digital-single-market/
At the end of each phase, the PCP R&D suppliers are evaluated according to the defined published evaluation process - in a fair and transparent process - and the best R&D suppliers are retained for the next phase.
Phase 0 – Preparation phase (until the submission deadline for tenders)
The preparation phase will involve an Open Market Consultation (OMC) (supporting the future preparation of the call for tenders) to ensure concrete alignment between the patient and clinical needs with the 7 leading clinical centres. After gathering all feedback from the OMC, the next PCP phase will start with the publication of the call for tenders from R&D suppliers. There will be 4 technical modules: pre-analytics/library preparation, sequencing, bioinformatics analysis, e-reporting/e-medication).
Phase 1 – Design of lots
Phase 2 – Prototypes of lots
Phase 3 – Full integrated NGS workflow
At the end of Phase 3, Instand-NGS4P will provide 2 fully integrated, standardized NGS workflows for routine diagnostics of common and rare cancers from adults to children.