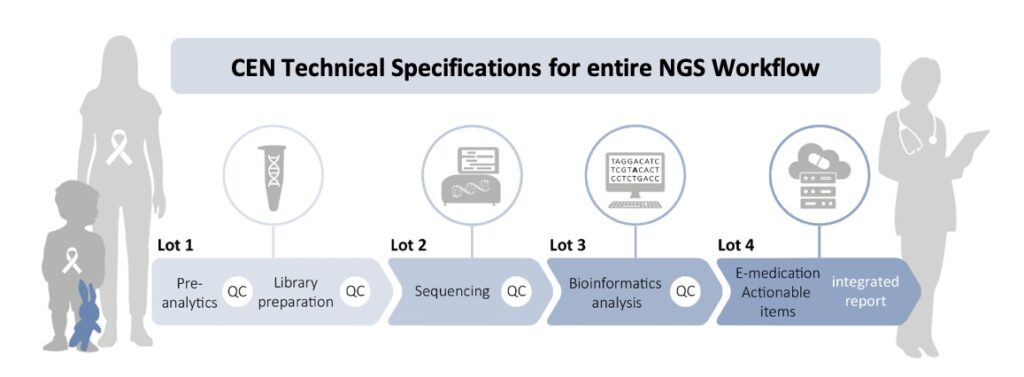
Analysis carried out by Instand-NGS4P´s WP7 of existing gaps in current standardization led to the proposal of 2 new Work Items for development in partnership with CEN/TC 140 “In vitro diagnostic medical devices” WG3 “Quality Management in the Medical Laboratory”:
00140151 - prCEN/TS XXX In vitro diagnostic Next Generation Sequencing (NGS) workflows - Part 1: Human DNA examination
00140153 - prCEN/TS XXX In vitro diagnostic Next Generation Sequencing (NGS) workflows - Part 2: Human RNA examination
A working draft ballot is open until the 14th of October to integrate the CEN/TC members and liaison partners before the subsequent survey of the TS draft. Instand-NGS4P has established liaisons with ISO/TC 276, ISO/TC 212, and ISO/TC 215/SC1. Commenting is possible via the National Standardization Bodies. The final TS vote will take place in 2023.
Commenting deadline: 14th October 2022